
So, using the information that we learned earlier, by stating the units in Standard Volumetric Flow Rate we are actually stating the Molar Flow Rate. We are stating the volumetric flow that would be present using standard conditions. Standard Liters per Minute (SLM) or Standard Cubic Centimeters per Minute (SCCM)). This is also the reasoning for the addition of the “S” or “Standard” at the start of the stated volumetric flow rate (e.g. The STP conditions are simply stated to define the standard volumetric flow rates of a substance IF it were an ideal gas at standard conditions. The substance may not even be in gas phase at 0☌.

Gas conditions are not brought to 0☌ and 760 Torr prior to running calibration of equipment. Our initial mass flow rate of 1 SLM of Nitrogen at 0☌ and 760 Torr would now be 1.074 SLM of Nitrogen at 20☌ and 760 Torr.Īn important item to note is that the STP conditions are not actually present during the calibration of mass flow meters and mass flow controllers. If we were to change to the second set of conditions, the number of moles present in the flow (Molar Flow Rate) would change, and our mass flow rate would thus change (based on the direct relationship between mass and moles). As I’ve discussed earlier, the 1 SLM must be referenced to an STP value, so we will use our assumed conditions of 0☌ and 760 Torr.

Suppose that we are looking to measure 1 SLM (Standard Liter per Minute) of Nitrogen gas. We will use the frequently referenced STP of 20☌ and 760 Torr for the second part of this example. Teledyne Hastings Instruments assumes STP of 0☌ and 760 Torr, but would prefer the customer to specify their STP conditions for the application. We’ll look at an example of the difference of STP conditions in a mass flow meter. This is based on the assumption that the measured gas is pure and not contaminated with any other gases. molar mass), we are able to calculate the mass of the volume or volumetric flow based on the number of moles present. Since the number of molecules of a gas and the mass are directly related for each gas type (i.e.
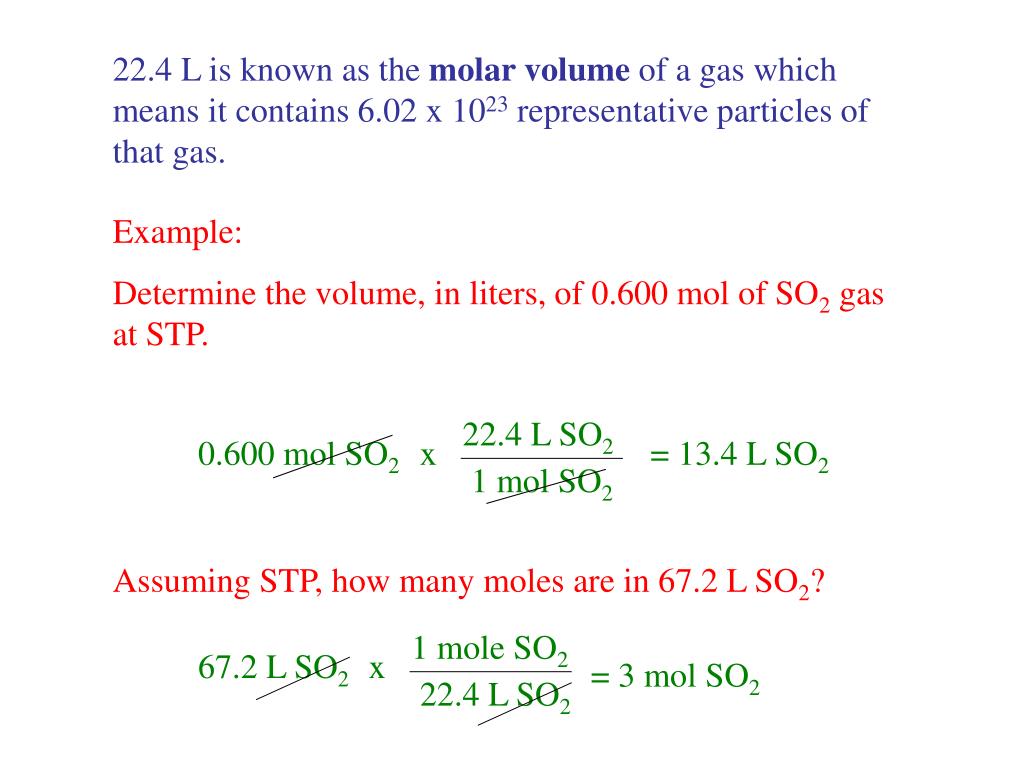
A mole is a number, just like one dozen is 12, so one mole is 6.02x10 23 molecules). Otherwise it is impossible for us to know “how much” of the substance (n) there is in the space (V) or flowing through the system.īut given all of this information do we actually end up with the mass flow? The actual quantification of this “how much” calculation is expressed in moles (n), which is an extremely large number of molecules of a gas stated as Avogadro’s number, equal to 6.02x10 23 (Don’t be scared by this value, though.

If we look at the ideal gas law that you may remember from a chemistry class school (PV = nRT), we understand that temperature (T) and pressure (P) must also be considered in the equation. Gases, however, ARE compressible and so the volume is only one factor in determining the amount of material being measured. This leads to a simple calculation using density with the already determined volume to find the mass present in the volume or the volumetric flow. When examining liquid flow instruments, we know that liquids are incompressible and thus the amount of a substance present is determined by the volume being used. Looking at an application with its established requirements, we often jump right to determining “what flow rate is required?” However, it is important to remember that mass flow applications using volumetric units must reference a standard temperature and pressure. As I go through the day looking at various mass flow applications, I often notice that it is very easy for users to overlook one of the crucial items required for calculating mass flow.


 0 kommentar(er)
0 kommentar(er)
